2023 Volume 48 Issue 5 Pages 227-241
2023 Volume 48 Issue 5 Pages 227-241
We conducted a two-year inhalation study of butyl methacrylate using F344/DuCrlCrlj rats and B6D2F1/Crl mice. Rats were exposed to 0, 30, 125 and 500 ppm (v/v) and mice were exposed to 0, 8, 30 and 125 ppm (v/v) using whole-body inhalation chambers. Non-neoplastic lesions developed in the nasal cavities of both rats and mice, but neoplastic lesions were not found. There was also a positive trend in the incidence of large granular lymphocytic (LGL) leukemia in the spleen of male rats. No changes were observed in female rats. Overall, there is some evidence of carcinogenicity in male rats, but there is no evidence of carcinogenicity in female rats. In male mice, there was a positive trend by Peto’s test in the incidence of hepatocellular adenomas, and the incidence of hepatocellular adenomas and hepatocellular carcinomas combined was significantly increased compared to the controls by Fisher’s exact test in the 30 ppm exposed male group. In female mice, the incidence of hemangiosarcoma in all organs combined showed a positive trend by Peto’s test. Therefore, there is some evidence of carcinogenicity in male mice, and there is equivocal evidence of carcinogenicity in female mice.
Butyl methacrylate (CAS No. 97-88-1) is a mono-functional alkyl ester of methacrylic acid. It is a colorless clear liquid with a boiling point of 160°C. This substance is soluble in ethanol and ethyl ether but is only slightly soluble in water. It has a number of uses, including the manufacture of resins, solvent coatings, and adhesives, and paper processing. Butyl methacrylate can also be produced during production of other alkyl esters of methacrylic acid and is also a breakdown product of longer chain alkyl methacrylates. Alkyl methacrylates are high production volume chemicals and the total amount of alkyl methacrylates (carbon side chains of 2 to 20 atoms) manufactured and imported by Japan was 20,000 to 30,000 tons in 2020 (Ministry of Economy, Trade and Industry of Japan, 2022). The total amount of butyl methacrylate that was released from various industrial sectors into the ambient air and public water in Japan in 2020 was 2.0 tons (Ministry of the Environment, Government of Japan, 2022).
A few studies on the toxicity of butyl methacrylate in rodents have been reported, but almost all of them were in-house non-peer reviewed publications. One inhalation study reported developmental toxicity when rats were exposed to 600 and 1200 ppm n-butyl methacrylate for 6 hr/day during days 6 to 20 of gestation (Saillenfait et al., 1999), and Deutsche Forschungsgemeinschaft (DFG) concluded that dermal exposure to n-butyl methacrylate can cause allergic skin reactions in eczema patients (DFG, 2001). However, no long-term toxicity or carcinogenicity studies have been carried out. In the present study, to provide basic data for health risk assessment of workers exposed to butyl methacrylate, male and female F344 rats and male and female B6D2F1 mice were exposed by whole body inhalation to butyl methacrylate for 2 years.
The present study was conducted in accordance with “Standards to be Observed by Testing Institutions” of Notification No.76 of the Ministry of Labour, Japan, September 1, 1988 (amendment: Notification No.13 of the Ministry of Labour, Japan, March 29, 2000), and in accordance with the Organisation for Economic Co-operation and Development (OECD) Good Laboratory Practice (OECD, 1998), and in accordance with the OECD Guideline for Testing of Chemicals 451 “Carcinogenicity Studies” (OECD, 2009), and in accordance with the Guideline for Proper Conduct of Animal Experiments (Science Council of Japan, 2006). This study was approved by the Animal Experiment Committee of the Japan Bioassay Research Center.
Test substanceReagent grade butyl methacrylate (purity > 99.8%) was obtained from Wako Pure Chemical Co., Ltd. (Osaka, Japan). Each lot of the butyl methacrylate used in the present study was verified by mass spectrometry. The stability of each lot of the butyl methacrylate used in the present study was confirmed by gas chromatography. No differences in the gas chromatography peaks of any of the lots of butyl methacrylate prior to and after use were observed. Additionally, butyl methacrylate in the inhalation exposure chambers was monitored using gas chromatography; no aberrant gas chromatographic peaks were detected.
AnimalsF344/DuCrlCrlj (SPF) rats and B6D2F1/Crl (SPF, IGS (International Genetic Standardization)) mice of both sexes were obtained at the age of 4 weeks from Charles River Japan, Inc. (Kanagawa, Japan). After 2 weeks of quarantine and acclimation, 50 rats and 50 mice of each sex were allocated through a stratified randomization procedure into body-weight-matched, butyl methacrylate exposure and control clean air-exposure groups: three butyl methacrylate-exposure groups and one control group. The animals were housed individually in stainless-steel wire hanging cages in stainless steel inhalation exposure chambers: 7.6 m3 for rats and 3.7 m3 for mice. Each exposure chamber was able to accommodate 100 rats or mice. The eight exposure chambers (four chambers for the rats and four chambers for the mice) were installed in a barrier system animal room. The chambers were maintained at a temperature of 23 ± 2°C and a relative humidity of 50 ± 20%, with 6 air changes per hour during the exposure periods and 12 air changes per hour during the non-exposure periods. There were 7–9 air changes per hour in the barrier system animal room. Fluorescent lighting in the animal room was automatically controlled to give a 12-hr light/dark cycle. All rats and mice had free access to a γ-irradiation-sterilized commercial pellet diet (CRF-LPF, Oriental Yeast Co., Ltd., Tokyo, Japan) and sterilized water.
Experimental designBased on growth suppression, anemia and airway irritation observed in our previous 13-week inhalation study of butyl methacrylate in rats and mice (unpublished), the maximum exposure concentrations for this 2-year study were set at 500 ppm for rats and 125 ppm for mice. Four groups of rats of each sex were exposed to butyl methacrylate vapor at 0 (clean air control), 30, 125 or 500 ppm (v/v), and the 4 groups of mice of each sex were exposed to butyl methacrylate vapor at 0 (clean air control), 8, 30 or 125 ppm (v/v), for 6 hr/day, 5 days/week for 104 weeks. The exposure results in the present study clearly met the maximum tolerated dose (MTD) criteria established by the National Cancer Institute (NCI) and IARC guidelines (Sontag et al., 1976; Bannasch et al., 1986). This is because the highest concentrations of 500 ppm in rats and 125 ppm in mice did not induce any life-threatening disorders or weight loss in this study.
Inhalation exposure to butyl methacrylateAirflows containing butyl methacrylate at the designated target concentrations were prepared using a vaporization technique: a saturated butyl methacrylate vapor-air mixture was generated by bubbling compressed clean air through butyl methacrylate liquid in a temperature-regulated glass flask (35°C) and then cooling the mixture by passage through a thermostatic condenser at 18°C. The cooled butyl methacrylate-air mixture was then introduced into a warming unit which was re-warmed to 23°C, which completely vaporized the butyl methacrylate contained in the vapor-air mixture. The butyl methacrylate vapor/air mixture was introduced into a spiral line mixer located above each chamber using a flow meter. In this mixer, the vapor/air mixture was diluted to the target concentration with clean air of controlled humidity and temperature and supplied to the inhalation chamber.
Butyl methacrylate concentrations in the chambers were monitored using gas chromatography every 15 min throughout the exposure period. The butyl methacrylate concentration in the inhalation exposure chambers for the 104 week study period is shown in Fig. 1. Butyl methacrylate concentrations were maintained at 0 (control) ppm, 29.9 ± 0.3 (mean ± SD) ppm in the 30 ppm chamber, 124.8 ± 0.7 ppm in the 125 ppm chamber, and 499.3 ± 3.5 ppm in the 500 ppm chamber of rats (Fig. 1A), and 0 (control) ppm, 8.0 ± 0.1 ppm in the 8 ppm chamber, 29.9 ± 0.3 ppm in the 30 ppm chamber, and 124.7 ± 0.7 ppm in the 125 ppm chamber of mice (Fig. 1B). Thus, the butyl methacrylate concentration in the inhalation exposure chambers was maintained with high accuracy at the target concentrations.
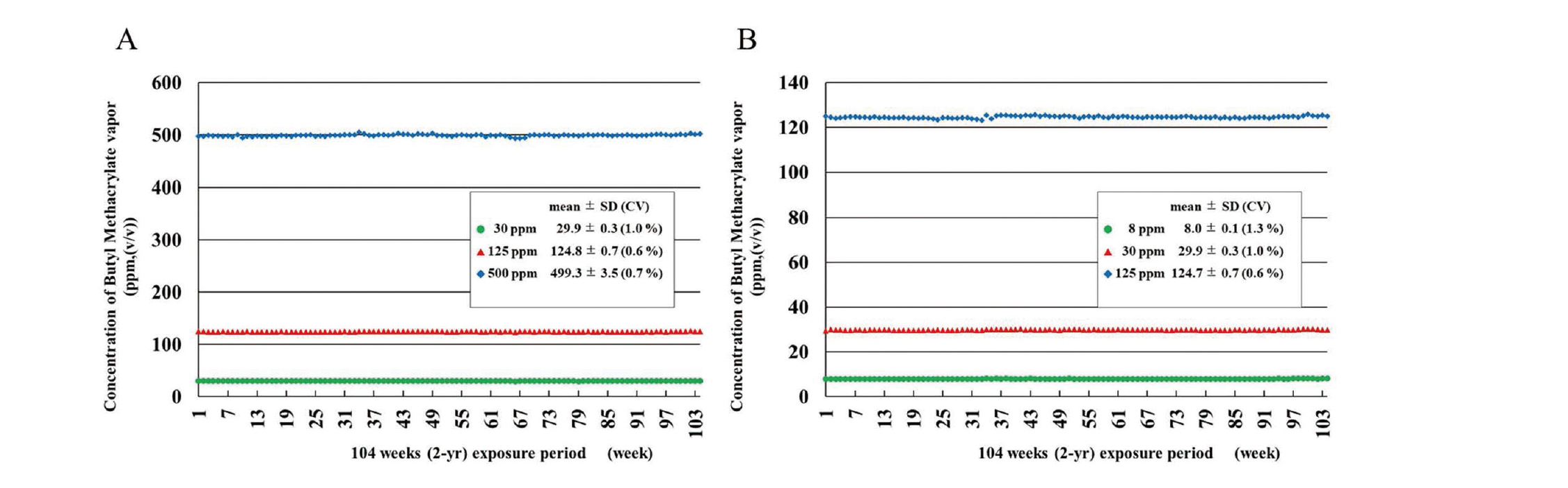
Butyl methacrylate concentrations throughout the 104 week experimental period. Butyl methacrylate vapor was confirmed to be generated at each target concentration throughout the exposure period (6 hr/day, 5 days/week, 104 weeks) in the rat inhalation exposure chambers (A) and in the mouse inhalation exposure chambers (B).
The animals were observed daily for clinical signs and mortality. Body weight and food consumption were measured once a week for the first 14 weeks and once every 4 weeks thereafter. All animals, including those found dead or moribund, received a complete necropsy. For hematology and blood biochemistry, blood was collected under anesthesia at the terminal necropsy after overnight fasting. The blood samples were analyzed using an automatic blood cell analyzer (ADVIA120, Bayer Co., Tarrytown, NY, USA) and a Hitachi 7080 chemistry analyzer (Hitachi, Ltd., Ibaraki, Japan) for blood biochemistry.
Organs were removed, weighed, and examined for macroscopic lesions at necropsy. All organs and tissues, including the entire respiratory tract (including the nasal cavity, pharynx and larynx), were examined histopathologically. The organs and tissues were fixed in 10% neutral buffered formalin. The nasal cavity was decalcified by immersion in formic acid-formalin solution prior to trimming and was transversely trimmed at three levels according to the procedure described in our previous paper (Nagano et al., 1997): at the level of the posterior edge of the upper incisor teeth (Level 1), at the incisive papilla (Level 2), and at the level of the anterior edge of the upper molar teeth (Level 3). The tissues were embedded in paraffin and 3 μm-thick sections were prepared and stained with hematoxylin and eosin (H&E). Nasal lesions were diagnosed with reference to the criteria set by the International Classification of Rodent Tumours (IARC, 1992).
Histopathological diagnosis was performed by pathologists certified by the Japanese Society of Toxicologic Pathology and peer reviewed by outside pathologists.
Statistical analysisThe incidences of neoplastic lesions were statistically analyzed by Fisher’s exact test. Positive trends for neoplastic incidence were analyzed using Peto’s test (Peto et al., 1980). Tumor incidence was also evaluated by comparison with incidences of the same tumor type from the Japan Bioassay Research Center (JBRC) historical control data. The use of historical control data for evaluation of tumor induction is described by Haseman et al. (1984, 1985, 1995). Historical control data for F344/DuCrlCrlj rats (SPF) were collected in 13 studies, excluding this study, with 649 males and 650 females. In the mouse study, the B6D2F1/Crl mice (SPF, IGS (International Genetic Standardization)) used in this study was the first time this strain of mice was used in our research center, and consequently, historical control data for this strain of mice are not available. Therefore, the historical control data for the B6D2F1/Crlj (SPF, non IGS) mouse strain, which is composed of 399 male and 400 female mice used in our research center, were used for reference in the present study. Incidences of non-neoplastic lesions were analyzed by Chi-square Test.
Body weight, organ weights, hematological and blood biochemical parameters were analyzed by Bartlett’s test to test whether the variance was homogeneous. When the variance was homogeneous, one-way ANOVA was used to test for statistical differences between groups, and when the variance was not homogeneous, the Kruskal-Wallis rank sum test was used. Statistical difference from the control group was analyzed by Dunnett’s multiple comparison test when the variance was homogeneous and Dunnett’s multiple comparison test by rank when the variance was not homogeneous.
Survival curves were plotted according to the Kaplan-Meier method, (Kaplan and Meier, 1958). The log-rank test (Peto et al., 1977) was used to test for statistically significant difference between butyl methacrylate-exposed rat and mouse groups of either sex and their respective controls.
Survival rates are shown in Fig. 2A and 2B and Table 1. The survival rates of males exposed to 500 ppm butyl methacrylate decreased during the late administration period compared with the male controls. Survival at the end of the 2-year exposure period in the 0 (Control), 30, 125 and 500 ppm exposed groups was 38 (76%), 41 (82%), 36 (72%) and 28 (56%), respectively, in the male groups, and 39 (78%), 38 (76%), 37 (74%) and 37 (74%), respectively, in the female groups.

Survival curves of male (A) and female (B) rats exposed to butyl methacrylate or clean air as a control for 2 years. Body weights of male (C) and female (D) rats exposed to butyl methacrylate or clean air as a control for 2 years.

There were no differences in clinical signs found between any butyl methacrylate-exposed groups of either sex compared to their respective controls.
The body weights in males exposed to 500 ppm butyl methacrylate were slightly, but significantly, lower than the control group from week 70 to week 104, and in females exposed to 500 ppm butyl methacrylate body weights were significantly decreased from week 42 to week 104 (Fig. 2C, D). However, the lowest body weight was 93% of the control in males and 92% of the control in females. The final body weights of the 30, 125 and 500 ppm-groups at the end of the 2-year exposure period were 99%, 99% and 94% of the controls for males and 98%, 104% and 92% of the controls for females (Table 1).
Food consumption in males exposed to 500 ppm butyl methacrylate was suppressed sporadically toward the end of the 2-year administration period and in females exposed to 500 ppm butyl methacrylate food consumption was suppressed sporadically in the latter half of the exposure period (data not shown).
Organ weights, hematology and blood biochemistryThe weights of the liver, kidney and spleen are shown in Table 1. Male rats exposed to 125 and 500 ppm had increased relative liver weights (percent of body weight) and increased absolute and relative kidney weights. Female rats exposed to 125 and 500 ppm had increased absolute kidney weights and females exposed to 500 ppm had increased relative liver and kidney weights.
Blood analysis is shown in Table 2. Red blood cell count, hemoglobin (Hb) and hematocrit (Hct) were decreased in the 125 and 500 ppm exposed males and mean corpuscular hemoglobin concentration (MCHC) was decreased in all exposed male groups. The reticulocyte ratio in males exposed to 125 and 500 ppm was increased. Total protein, albumin, total cholesterol, and sodium were decreased, and potassium, chloride and inorganic phosphorus were increased in males exposed to 500 ppm. Urea nitrogen was increased in 125 and 500 ppm exposed males.

In female rats, there were no changes in hematology or blood biochemistry (data not shown).
PathologyMacroscopic observation found enlargement of the spleen, pleural fluid in the thoracic cavity, and subcutaneous masses in 500 ppm exposed males and white zones in the lungs of 500 ppm exposed females.
Histopathologically, there was a positive trend in the incidence of large granular lymphocytic (LGL) leukemia in the spleen of butyl methacrylate-exposed males by Peto’s test (Table 3, Fig. 3, 4). In addition, the incidence of LGL leukemia in the spleen of male rats exposed to 125 and 500 ppm (125 ppm: 22%; 500 ppm: 28%) was higher than the JBRC historical controls (61/649: range 4–14%).


LGL leukemia, Spleen, Male rat, 500 ppm.
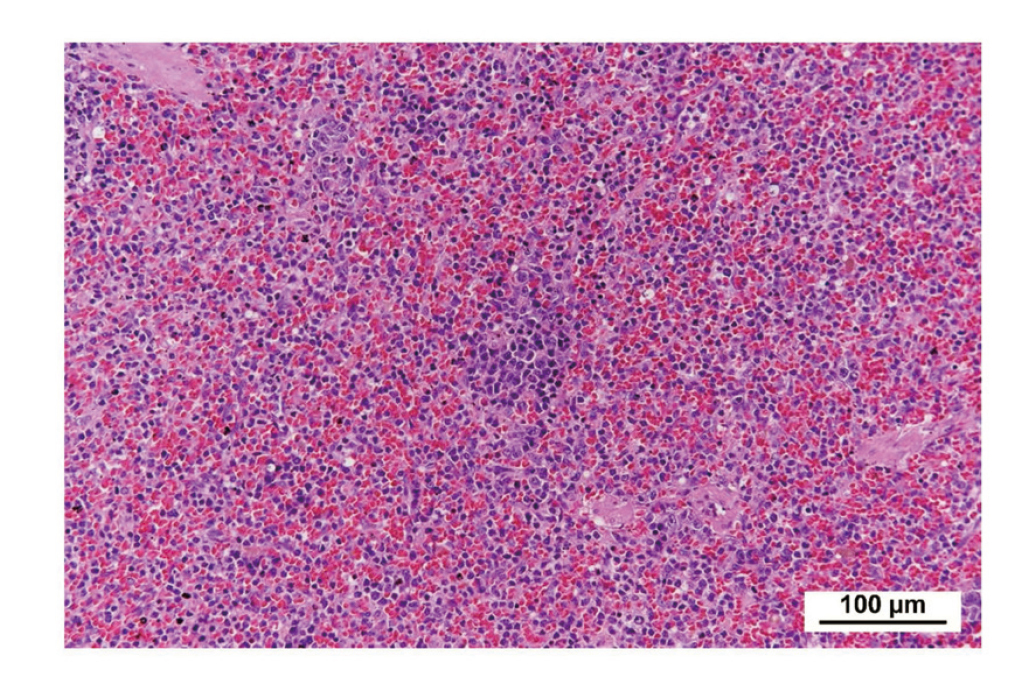
High magnification of Fig. 3.
There was a positive trend in the incidence of fibroma in the subcutis in butyl methacrylate-exposed males by Peto’s test (Table 3). However, the incidence of fibroma in the subcutis in exposed males was within the range of the JBRC historical controls (75/649: range 6–16%). Interstitial cell tumors in the testis, which is commonly found in aged male rats, also had a positive trend in incidence by Peto’s test (Table 3). However, the incidence was within the range of the JBRC historical controls (531/649: range 72–98%).
In females, there was no increase in the incidence of LGL leukemia in the spleen. Fibroadenoma in the mammary gland had a positive trend in incidence by Peto’s test (Table 3). However, the incidence of fibroadenoma in the mammary gland in the exposed groups was within the range of the JBRC historical controls (75/650: range 6–20%). There was also a significant increase in the combined incidences of C-cell adenoma and C-cell carcinoma in the thyroid of 125 ppm exposed females by Fisher’s exact test, but there was no positive trend in incidence by Peto’s test (Table 3). In addition, the incidence was within the range of the JBRC historical controls (84/650: range 2–26%).
Exposure-related non-neoplastic lesions were primarily found in the nasal cavity of males and females (Table 4). In the nasal respiratory epithelium, squamous cell metaplasia was increased in 500 ppm exposed males with slight to moderate severity and increased in 125 and 500 ppm exposed females with slight severity. In the olfactory epithelium, basal cell hyperplasia with slight severity was increased in 500 ppm exposed males and females. Regeneration of the olfactory epithelium was increased in 500 ppm exposed males. Atrophy with slight to moderate severity, and deposition of pigment in the olfactory epithelium with slight severity were increased in all exposed males and females; deposition of pigment was mainly observed at the basal side of the olfactory cell layer. Necrosis in the olfactory epithelium was observed in 1 male exposed to 125 ppm, 4 males exposed to 500 ppm, and 1 female exposed to 500 ppm. Eosinophilic changes were reduced and/or attenuated in the respiratory epithelium and olfactory epithelium.
In addition, there was an increase in the incidence of increased hematopoiesis in the bone marrow of 125 and 500 ppm exposed male rats, but not in female rats (Table 4). There were no exposure-related non-neoplastic lesions in any other organ/tissue.
Mouse study Survival, body weight, food consumption and clinical observationsSurvival rates are shown in Fig. 5A, 5B and Table 5. The survival rates of males exposed to 8 and 125 ppm butyl methacrylate were decreased compared with the controls. The survival rates of the female mice exposed to butyl methacrylate was comparable to the control group throughout the 2-year exposure period. Survival at the end of the 2-year exposure period in the 0 (Control), 8, 30, and 125 ppm exposed groups was 45 (90%), 35 (70%), 41 (82%) and 37 (74%), respectively, in the male groups, and 36 (72%), 32 (64%), 33 (66%), and 33 (66%), respectively, in the female groups.

Survival curves of male (A) and female (B) mice exposed to butyl methacrylate or clean air as a control for 2 years. Body weights of male (C) and female (D) mice exposed to butyl methacrylate or clean air as a control for 2 years.

No differences in clinical signs were found between any butyl methacrylate-exposed groups of either sex and their respective controls. The body weights in the exposed male and female groups were similar to those of the control groups: while the body weights of males and females exposed to 125 ppm butyl methacrylate were significantly suppressed from the 3rd week to the 82nd week of the exposure period compared to their controls, the lowest body weight was 92% of the control in males and 95% of the control in females (Fig. 5C, D). After week 82, body weights recovered to the control group level. The final body weights of the 8, 30 and 125 ppm-groups at the end of the 2-year exposure period were 102%, 103% and 100% of the controls in males and 102%, 101%, and 100% of the controls in females (Table 5).
Food consumption was suppressed in males exposed to 125 ppm during most of the 2-year exposure period, and in females exposed to 125 ppm food consumption was suppressed until week 30th of the exposure period (data not shown).
Organ weights, hematology and blood biochemistryThe weights of the liver, kidney and spleen are shown in Table 5. The absolute and relative weights of the spleen were significantly decreased in male mice exposed to 125 ppm. Other organ weights did not differ from the controls in any of the male or female groups.
Blood analysis of male mice is shown in Table 6. Hemoglobin (Hb) and mean corpuscular hemoglobin concentrations (MCHC) were increased in males exposed to 125 ppm, and the white blood cell count and reticulocyte ratio were decreased in males exposed to 125 ppm. Albumin and the albumin-globulin ratio were increased in the 30 and 125 ppm male groups, and glucose was increased in the 125 ppm exposed male group. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), creatinine kinase (CK), urea nitrogen, sodium and inorganic phosphorus were decreased in the 125 ppm male group.

In female mice, blood analysis of mean corpuscular volume (MCV) was increased in the 8 and 125 ppm groups and mean corpuscular hemoglobin (MCH) was increased in all exposed groups. There were no changes in blood biochemistry (data not shown).
PathologyMacroscopic observation found no exposure-related changes in either the male or female groups.
In male mice, there was a positive trend in the incidence of hepatocellular adenomas (Fig. 6) by Peto’s test (Table 7). The combined incidence of hepatocellular adenomas and hepatocellular carcinoma (Fig. 7) was increased in 30 ppm but not 125 ppm exposed males by Fisher’s test, and there was no positive trend by Peto’s test. Histiocytic sarcoma was observed in the subcutis, liver, epididymis and peritoneum, and there was a positive trend in the incidence in all sites combined by Peto’s test.
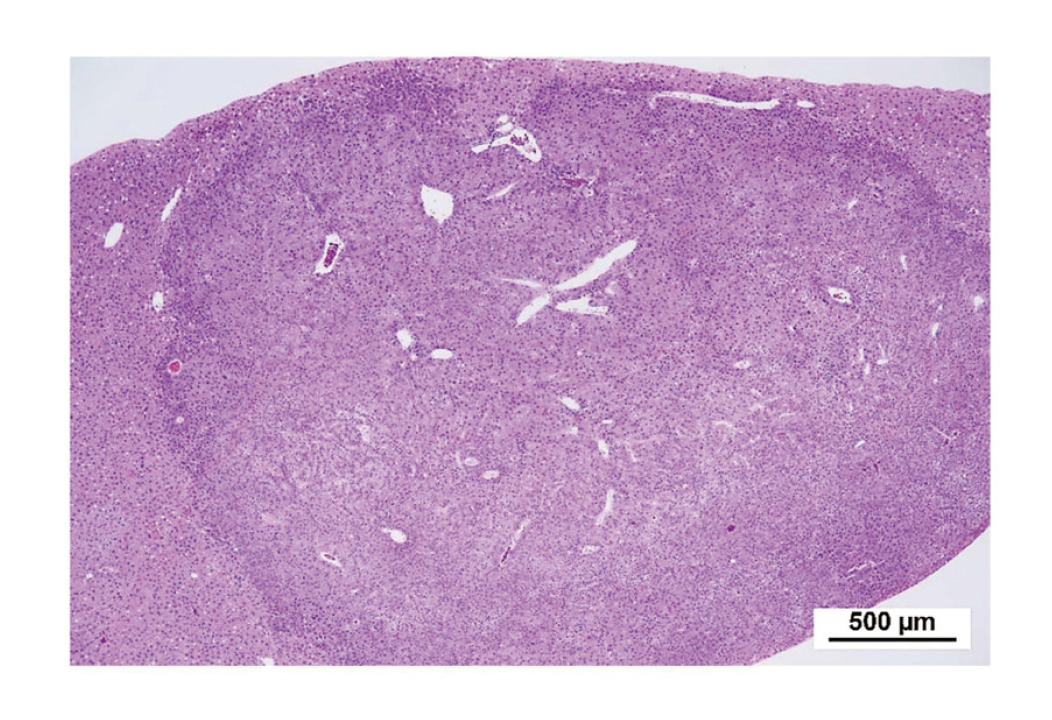
Hepatocellular adenoma, Liver, Male mouse, 500 ppm.


Hepatocellular carcinoma, Liver, Male mouse, 500 ppm.
In females, there was a positive trend in the incidence of adenomas in the anterior lobe of the pituitary gland by Peto’s test. Hemangiosarcomas were found in the subcutis, bone marrow, spleen, liver, uterus, peritoneum and retroperitoneum, and there was a positive trend in the incidence in all sites combined by Peto’s test.
Exposure-related non-neoplastic lesions were primarily found in the nasal cavity and nasopharynx of males and females (Table 8). In the olfactory epithelium in the nasal cavity, respiratory metaplasia was increased in 125 ppm exposed males and 30 and 125 ppm exposed females. Regeneration was increased in all exposed male groups and in the 8 and 30 ppm exposed female groups. Atrophy was increased in 125 ppm exposed males and 30 ppm exposed females. Olfactory lesions were found dorsally at the middle to posterior area (levels 2 and 3). In the olfactory gland and/or nasal gland in the nasal cavity, respiratory metaplasia was significantly increased in 125 ppm exposed males and 30 and 125 ppm exposed females. Respiratory metaplasia was found in level 2 at the boundary of the olfactory epithelium and the nasal turbinate and at level 3 in the dorsal wall and the ethmoturbinate. Eosinophilic change was observed in the respiratory epithelium and olfactory epithelium in the nasal cavity and in the nasopharynx. In the nasal respiratory epithelium, eosinophilic change was increased in 125 ppm exposed males and was of slight to moderate severity, and in females eosinophilic change was found in all animals in the control group, however, the severity of eosinophilic change was increased in the 125 ppm exposed female group. In the nasal olfactory epithelium eosinophilic change was increased in 125 ppm exposed males and females. In the nasopharynx, eosinophilic change was increased in 125 ppm exposed males with slight severity and in 30 and 125 ppm exposed females with slight to moderate severity.

In addition, non-neoplastic lesions were also found in the spleen, stomach and uterus (Table 8). In the spleen, the severity of follicular hyperplasia was increased in females exposed to 30 and 125 ppm, and the incidence of hemosiderin deposition was increased in the 125 ppm exposed female group with slight to moderate severity. In the glandular stomach, the incidence of epithelial hyperplasia was decreased in males and increased in females exposed to 125 ppm. In the uterus, hyperplasia in the gland was increased with slight severity in all exposed groups and the increase in the 30 ppm exposed group was statistically significant. There were no exposure-related non-neoplastic lesions in other organs.
There were no differences in clinical signs found between any butyl methacrylate-exposed group of rats and mice of either sex compared to their respective controls in this study. For rats, the terminal survival rate was decreased only in males exposed to 500 ppm butyl methacrylate. Body weights in the exposed groups were within 10% of the body weights of the controls throughout the 2-yr study period. Therefore, the highest dose of butyl methacrylate used in the present study met the MTD criteria.
In rats, there was a positive trend in the incidence of LGL leukemia in the spleen of butyl methacrylate-exposed males by Peto’s test. In addition, the incidences of LGL leukemia in the spleen of male rats exposed to 125 and 500 ppm (125 ppm: 22%; 500 ppm: 28%) were higher than the JBRC historical controls (61/649: range 4–14%). Therefore, exposure to butyl methacrylate was concluded to increase the incidence of LGL leukemia in the spleen of male rats.
In male rats, there was also a positive trend by Peto’s test in the incidences of fibroma in the subcutis and interstitial cell tumor in the testis of butyl methacrylate-exposed groups. However, the incidences of these lesions were within the ranges of the JBRC historical controls, indicating that the increased incidences were not caused by butyl methacrylate exposure.
In female rats, there was the positive trend by Peto’s test in the incidence of fibroadenoma in the mammary gland, and an increased incidence by Fisher’s exact test in the combined incidences of C-cell adenoma and C-cell carcinoma in the thyroid. However, the incidences of these tumors in 500 ppm exposed females (fibroadenoma: 9/50; C-cell adenoma plus C-cell carcinoma: 8/50) was within the range of JBRC historical controls (fibroadenoma: 75/650, range 6–20%; C-cell adenoma plus C-cell carcinoma: 84/650, range 2–26%). Therefore, these lesions could not be concluded to be exposure related.
In male mice, there was a positive trend by Peto’s test in the incidence of hepatocellular adenomas, and the combined incidence of hepatocellular adenomas and hepatocellular carcinomas was significantly increased in the 30 ppm exposed group by Fisher’s exact test. However, the incidence of hepatocellular adenoma in the control group was 23/50 mice while the incidence of hepatocellular adenoma in the group exposed to 125 ppm was 24/50 mice. In addition, the incidence of hepatocellular adenomas and hepatocellular carcinomas combined was not increased in the 125 ppm group (31/50) compared to the control group (32/50), and there was no positive trend by Peto’s test in the combined incidence of hepatocellular adenomas and hepatocellular carcinomas in butyl methacrylate-exposed male mice. One possibility is that poor nutritional status suppressed the development of liver tumors in male mice: the body weights of males exposed to 125 ppm butyl methacrylate were significantly suppressed compared to controls from the 3rd week to the 82nd week of the exposure period. This suggests that the incidence of hepatocellular adenomas and carcinomas in the 125 ppm exposed group is unlikely to be relevant and that the data of the controls, the 8 ppm exposure group and the 30 ppm exposure group should be used for evaluation of the carcinogenicity of butyl methacrylate in the male mouse liver. Using these groups, we concluded that the increase in hepatocellular adenoma was possibly caused by exposure to butyl methacrylate and the increase in hepatocellular adenoma and carcinoma combined was caused by exposure to butyl methacrylate.
There were also positive trends by Peto’s test in the incidence of histiocytic sarcoma in all organs combined in male mice, adenomas in the pituitary gland in female mice, and hemangiosarcomas in all organs combined in female mice. Since we have not used B6D2F1/Crl (SPF, IGS: International Genetic Standardization) mice in our previous mouse carcinogenicity studies, there are no historical control data for this strain of mouse. However, the historical control data for B6D2F1/Crlj (SPF, non IGS) mice (399 males and 400 females) are as follows: incidence of histiocytic sarcoma in all organs combined in males is 7.8%, range 2%–12%; incidence of adenomas in the anterior lobe of the pituitary gland in females is 12.8%, range 4%–20%; incidence of hemangiosarcomas in all organs combined in females is 3.0%, range 0%–6%. The incidences of hepatocellular adenomas in males exposed to 30 ppm (30/50), hepatocellular adenoma and hepatocellular carcinoma combined in males exposed to 30 ppm (41/50), and hemangiosarcomas in all organs in females (4/50) were higher than the historical control data for B6D2F1/Crlj (SPF, non IGS) mice. The incidence of histiocytic sarcoma in all organs combined in males (3/50) and the incidence of adenomas in the anterior lobe of the pituitary gland in females (6/50) were not higher than the historical control data for B6D2F1/Crlj (SPF, non IGS) mice.
Overall, based primarily on the fact that the increased incidence of hepatocellular adenoma and carcinoma combined in the 30 ppm exposed males was statistically significant by Fisher’s Exact Test, it is concluded that the increase in hepatocellular adenoma and carcinoma combined was caused by exposure to butyl methacrylate. Based on Peto’s test and the historical control data for B6D2F1/Crlj (SPF, non IGS) mice, it is concluded that the increases in hepatocellular adenomas in male mice and hemangiosarcomas in all organs in female mice were possibly caused by exposure to butyl methacrylate, and that the increases in histolytic sarcomas in all organs in male mice and adenomas in the anterior lobe of the pituitary gland in female mice cannot be concluded to be caused by exposure to butyl methacrylate.
The airway irritant properties of butyl methacrylate have been reported in animal studies (ECETOC, 1997). In the present study, various non-neoplastic pathological changes were observed in the nasal cavity in rats and mice exposed to butyl methacrylate (see Tables 4 and 8). In mice, these changes tended to occur at lower exposure concentrations in females than in males. Notably, while non-neoplastic lesions were observed in various sites of the nasal cavity, including lesions commonly associated with tumorigenesis such as hyperplasia and metaplasia, neoplastic lesions were not observed in the nasal cavities of any of the exposed animals.
In the present study, sex differences were observed in butyl methacrylate carcinogenicity and chronic toxicity. Accordingly, we examined carcinogenicity and chronic toxicity from the perspective of inhalation specific intake and metabolism of butyl methacrylate. The chemical intake of butyl methacrylate into the body was calculated based on Bide et al. (2000). Since body weight and respiration are closely related, the average body weight of rats and mice during the study period was used (male rats 338.5 g; female rats 194.5 g; male mice 37.9 g; female mice 27.7 g). The formula for determining the tidal volume of the animals was Vm = 0.499 x BW0.809 by Bide et al. (2000). The 6-hr chemical intake of butyl methacrylate = Vm x 360 (min) x exposure concentration (ppm converted to mg/m3). Using exposure concentrations of 500 ppm for rats and 125 ppm for mice into the above formula yields values 217.5 and 138.9 mg/body weight for male and female rats, and 9.3 and 7.2 mg/body weight for male and female mice. Thus, even at the same exposure concentrations, the converted doses (chemical intake of butyl methacrylate) are higher in males than in females. Thus, the difference in toxicity observed between males and females may simply be due to the higher intake of butyl methacrylate in male rats and mice.
Regarding metabolism, there are several metabolic pathways for butyl methacrylate. First, butyl methacrylate is thought to be rapidly metabolized to methacrylic acid and n-butanol by carboxylesterase in the olfactory epithelium of the nasal cavity (National Center for Biotechnology Information (NCBI), 2022; OECD, 2004). Methacrylic acid is then metabolized by cytochrome P450 to the cytotoxic 2,3-epoxymethacrylic acid (Reichl et al., 2010). On the other hand, n-butanol is thought to be metabolized to butyraldehyde in the rat liver (Teschke et al., 1975). Cytochrome P450 activity has been shown to be higher in males than in females in rats and mice (Su et al., 1996; Kato and Yamazoe, 1992), and consequently, the formation of 2,3-epoxymethacrylic acid after inhalation exposure to butyl methacrylate should be greater in males. Therefore, it is logical to expect that inhalation exposure to butyl methacrylate would be more toxic in males than females. However, the toxicity of butyl methacrylate and 2,3-epoxymethacrylate has not been compared, and it is unclear whether the toxicity observed in the present study was due to butyl methacrylate, its metabolites, or a combination of these compounds. Carcinogenesis is a complex interplay of various factors, and further research is needed on the carcinogenicity of butyl acrylate, including sex differences observed in rats and mice.
Since genotoxicity studies have shown negative results, butyl methacrylate is considered to be non-genotoxic (Ministry of Health & Welfare of Japan, 1998; Albertini et al., 2017; Zeiger et al., 1987; Johannsen et al., 2008). We also reported the negative results in Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537 and the Escherichia coli strain WP2uvrA/pKM101 with or without an exogenous metabolic activation system (JETOC, 2000). It was also reported that butyl methacrylate did not induce structural chromosomal aberrations or polyploidy in Chinese Hamster Lung (CHL) cells with or without an exogenous metabolic activation system (Ministry of Health & Welfare of Japan, 1998).
The developmental toxicity of butyl methacrylate was investigated in Sprague-Dawley rats exposed by inhalation for 6 hr/day between days 6 and 20 of gestation. The exposure concentrations were 0, 100, 300, 600 and 1200 ppm. Exposure to 300 and 600 ppm butyl methacrylate resulted in mild maternal toxicity, evidenced by a transient decrease in weight gain and a slight and transient decrease in food consumption. Maternal toxicity was more pronounced in the highest butyl methacrylate exposure group of 1200 ppm, which was also observed as a reduction in weight gain. Fetal toxicity, evidenced by a significant decrease in fetal body weight, appeared at 600 ppm butyl methacrylate. Developmental toxic effects were clearly dissociated from maternal toxicity (Saillenfait et al., 1999).
DFG concluded that dermal exposure to n-butyl methacrylate can cause allergic skin reactions in eczema patients (DFG, 2001). Other agencies, such as the International Agency for Research on Cancer (IARC), American Conference of Governmental Industrial Hygienists (ACGIH) and the Japan Society for Occupational Health (JSOH) have not evaluated allergic reactions to n-butyl methacrylate.
In this study, we conclude that there is some evidence for potential carcinogenicity of butyl methacrylate in male rats, and no evidence for carcinogenicity in female rats. In mice, we conclude that there is some evidence for potential carcinogenicity of butyl methacrylate in males, and there is equivocal evidence for potential carcinogenicity in females.
Extrapolation of these carcinogenicity conclusions in rats and mice to humans should be decided based on the weight of evidence concept of “the mode of action” for carcinogenesis.
The present study was contracted and supported by the Ministry of Health, Labour and Welfare, Japan. We also acknowledge the efforts of member of JBRC.
Conflict of interestThe authors declare that there is no conflict of interest.